
Sections
Highlight

Julia Fernández
Friday, 16 February 2024, 17:46
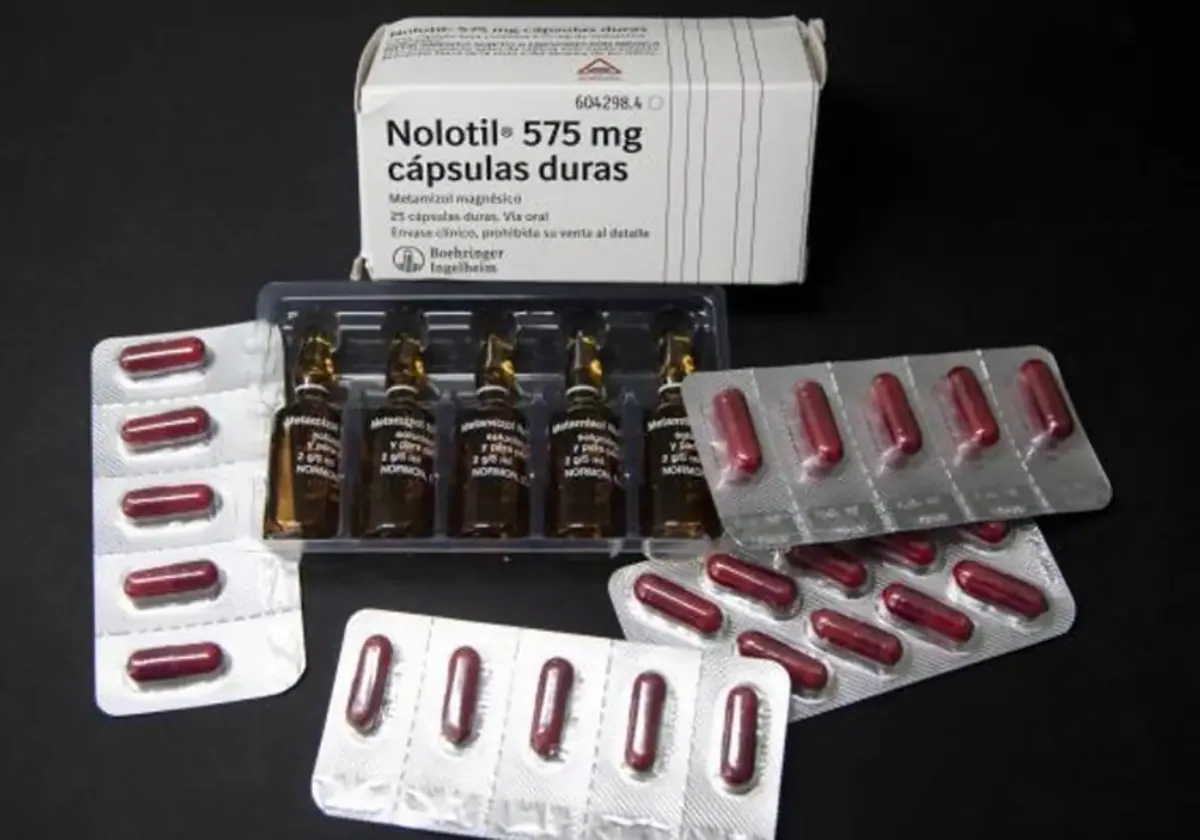
A complaint filed against the ministry of health and the Spanish medicines agency AEMPS concerning the prescribing of the painkiller Nolotil is now being studied by the Audiencia Nacional, Spain's high court. The drug is banned in the UK where the population is considered more at increased risk of agranulocytosis, a serious adverse effect that can lead to death as detailed in the product's information on side-effects.
ADAF, an association formed for sufferers of the negative consequences of the use of medications, acted following the death of several patients who were prescribed Nolotil (one of the brands that markets the active ingredient metamizole) in Spain. The lawsuit, filed last November and which in January reached the attorney general's office, accuses the Spanish authorities of inactivity "in real, adequate, updated and responsible pharmacovigilance" by not taking this information into account.
According to medical sources, agranulocytosis occurs in very rare cases in Spain (around one case per 10,000) and is related to the period of time over which the patient takes metamizole. However, the prescription of this drug should be undertaken only when it is possible to follow up on the patient. For this reason, the ADAF considers that a crime of serious recklessness due to “negative” action has been committed, which has caused “damage and even death in certain patients”.
According to data from the group, the drug has caused 45 deaths with 125 other patients affected in recent years. ADAF has provided information from medical records in Spain of “a broad list of people” who have been affected (both Spanish nationals and international patients) with evidence of the damage caused by this drug and of the communication of adverse events to AEMPS and private companies. Legal sources consulted by Europa Press, which has had access to the lawsuit, indicate that there are several complaints from individuals in this regard that are being analysed in parallel in the Madrid prosecutor's office.
ADAF alleges that many officials of the AEMPS and of the different health administrations in charge of pharmacovigilance in Spain, “are not doing their job responsibly ... by not alerting the medical and health community to imperative aspects such as the immediate prohibition of the prescription in Spain of products containing metamizole to foreigners in whose countries it is prohibited: United States, United Kingdom, Ireland, Sweden, Japan, Australia, etc”.
On 1 December, with the case in the media, AEMPS issued a statement announcing that it maintained recommendations on metamizole to prevent the risk of agranulocytosis, after having carried out a new review on this drug. Likewise, the Spanish society of primary care pharmacists, SEFAP, insisted that “there is no new evidence that changes the risk profile of the drug”.
The group that brought the lawsuit considers that the authorities “look the other way without doing anything” and that many people “are dying and suffering very serious adverse reactions". Citing alleged criminal actions, the group said they had detected “serious recklessness, the omission or intentional concealment of safety information of a drug, the failure to carry out adequate and responsible pharmacoepidemiological studies, the non-activation of safety and caution protocols, among other aspects”.
“Why in Spain, despite indications and alerts in pharmacovigilance, is it chosen to issue a small information note where AEMPS and medical and pharmaceutical companies know that it is not being complied with?” the group also said.
The group highlights that the grave matter especially affects the British population and that there has been a “lack of attention or disregard for published independent studies warning of under-reporting and danger, of the error in the prescription in general”.
In 2018 the metamizole data sheet was reviewed by the AEMPS when receiving reports of cases of agranulocytosis, especially in British patients. The largest British populations in Spain are located in Andalucía, Levante (Costa Blanca), the Balearic Islands and the Canary Islands. The conclusion of the study was that the increase in cases was parallel to the growth in the user of this painkiller, the most widely used treatment of its kind in Spain.
Experts have considered that there were no data to confirm or rule out the incidence of genetic factors to explain possible differences in the appearance of this disease according to country of origin. Nevertheless, it was recommended not to prescribe Nolotil or Buscapina Compositum (two brands under which the active ingredient is marketed) to tourists, the ADAF said.
Publicidad
Publicidad
Publicidad
Publicidad
Esta funcionalidad es exclusiva para registrados.
Reporta un error en esta noticia

Debido a un error no hemos podido dar de alta tu suscripción.
Por favor, ponte en contacto con Atención al Cliente.

¡Bienvenido a SURINENGLISH!

Tu suscripción con Google se ha realizado correctamente, pero ya tenías otra suscripción activa en SURINENGLISH.
Déjanos tus datos y nos pondremos en contacto contigo para analizar tu caso

¡Tu suscripción con Google se ha realizado correctamente!
La compra se ha asociado al siguiente email
Comentar es una ventaja exclusiva para registrados
¿Ya eres registrado?
Inicia sesiónNecesitas ser suscriptor para poder votar.