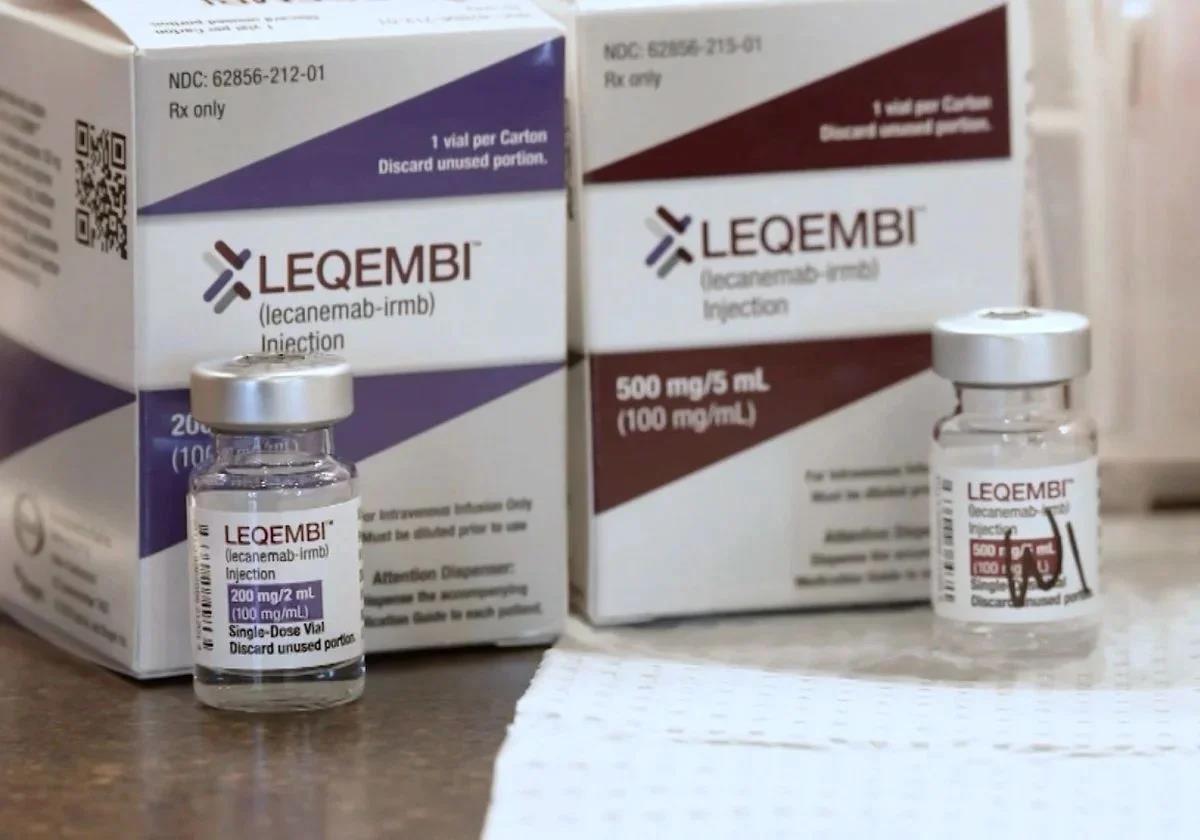
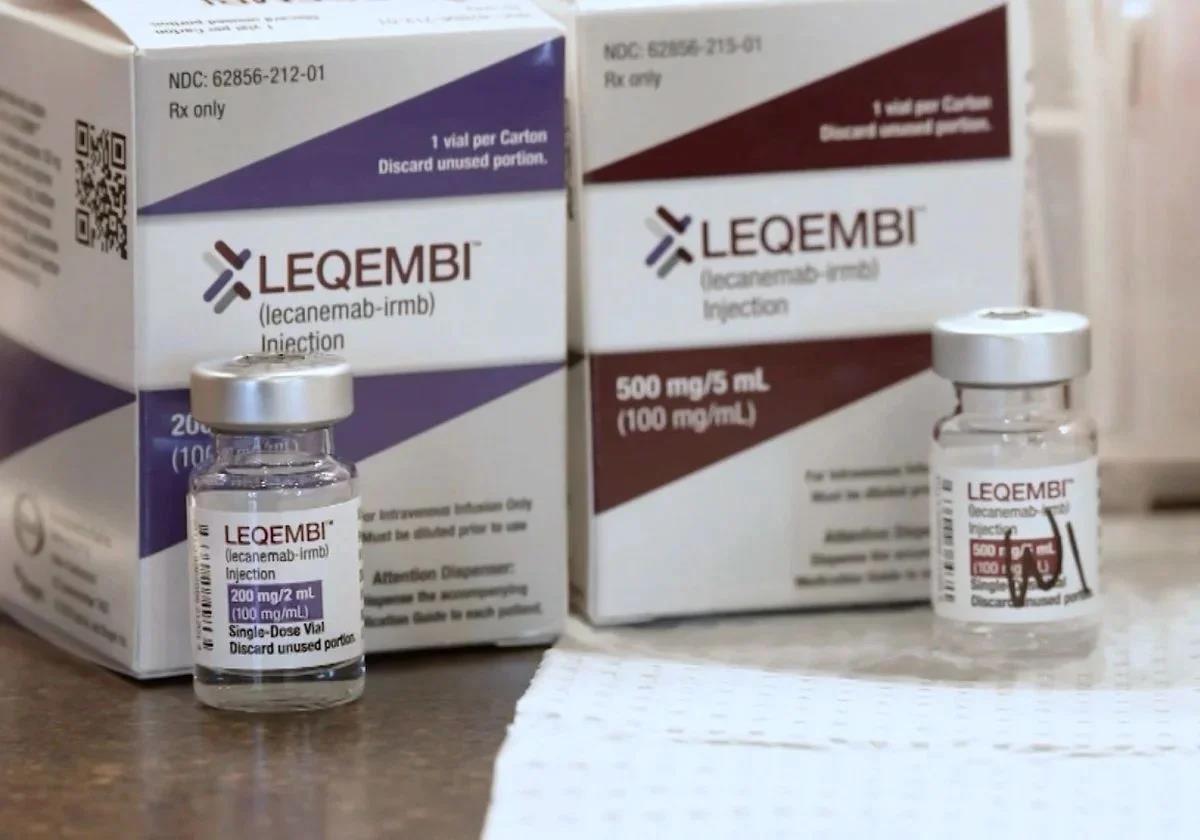
Sections
Highlight
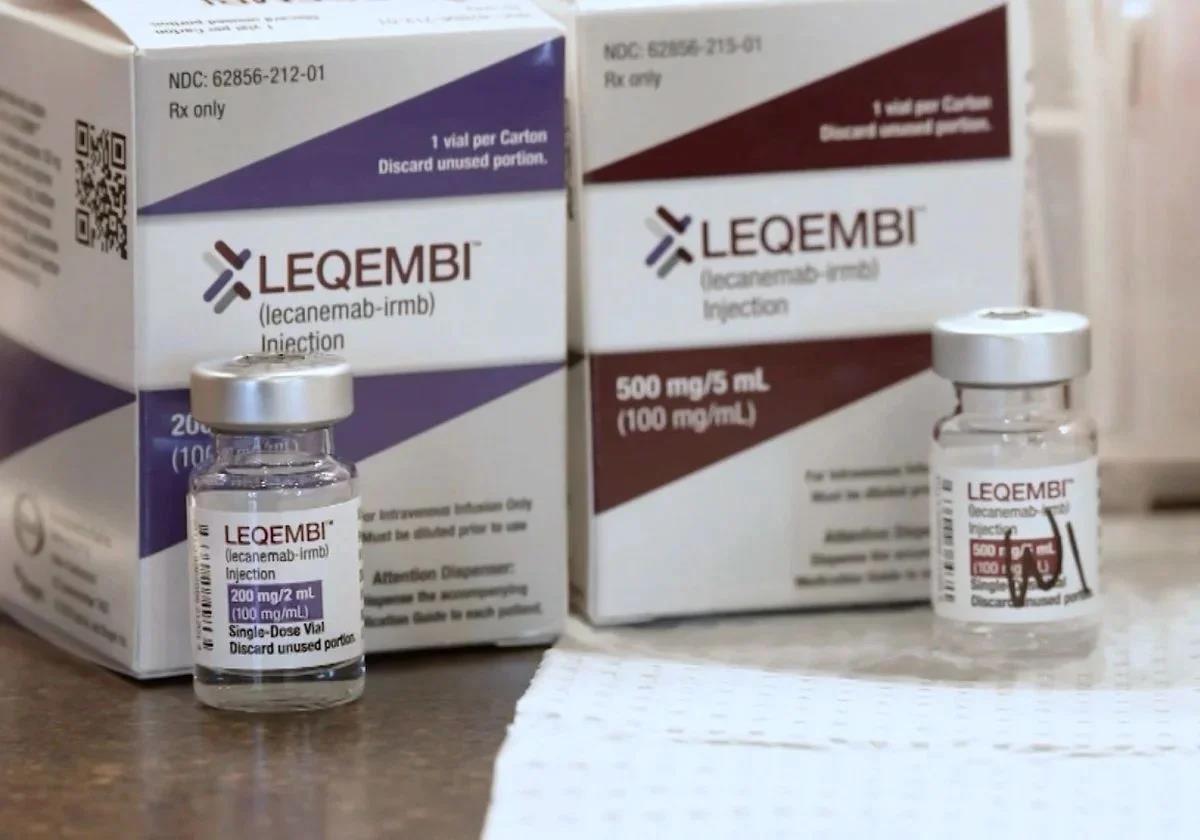
N. Ramírez de Castro / ABC
Madrid
Thursday, 17 April 2025, 10:57
After more than two decades without significant advances, Europe has given the green light to Leqembi (lecanemab) - the first drug that promises to slow the deterioration from Alzheimer's disease. The European Commission's decision, based on a new positive report from the European Medicines Agency, allows the first phase of the drug's financing in Spain. The news has brought hope to patient associations and institutions involved in Alzheimer's research.
Lequembi is not a cure for Alzheimer's, but it is capable of slowing cognitive decline in patients in the early stages of the disease. With that said, it would not improve memory or cognitive skills if they have already been lost, but it could halt progression.
Will patients notice? Clinical trials have seen a range of changes: from subtle to adjustments that allow patients to preserve their autonomy, communication skills and quality of life for months or even years.
Not all Alzheimer's patients are eligible for the administration of lecanemab. Europe has authorised it for those who have only one copy of the ApoE4 gene - a genetic variant that increases the risk of developing the disease. Another group of people that can benefit from the drug are those with no copies of the gene but with presence of beta-amyloid plaques in the brain - a toxic protein linked to the disease. In their case, fortnightly intravenous infusion will clear the beta-amyloid plaques that build up in the brain.
The road to authorisation has not been straightforward. Both the European Medicines Agency and the US Medicines Agency initially rejected Lequembi, because they considered that the risks could outweigh the potential benefits. During the research phases, cases of swelling and hemorrhaging in the brain were detected among trial participants. These were mild cases that resolved on their own, but such side effects could have fatal consequences. As a result, the FDA (the US federal agency for public health protection) required a 'black box warning' (the highest level) on the drug's label, highlighting the risk of triggering "serious and life-threatening reactions".
It is now known that the risk of brain haemorrhage is related to taking anticoagulants - a common treatment for older people - at the same time as lecanemab.
In November 2024, the European Medicines Agency also changed its decision, which led to the European Commission's approval, although with strict criteria in all member countries. In particular, countries must ensure that all healthcare professionals, as well as patients who will use Lequembi, have access to information about its risks.
The EU has not announced the price of Lequembi, although it is expected to be high. In the US, the cost of treatment is estimated at 26,500 dollars a year. In Europe, it will be up to each member state to decide on the price and healthcare reimbursement.
The Fundación Pasqual Maragall and the Hub Alzhéimer Barcelona believe that the European approval is "a milestone in biomedical innovation", the result of decades of international collaboration. "We are aware that this drug is not free of side effects, but the most important thing is that, for the first time, people with Alzheimer's and their families will be able to decide, with medical support and plenty of information, if they want to access this new treatment," said the joint statement.
The Hub Alzhéimer Barcelona, which joins together six centres specialising in the research and treatment of this neurodegenerative disease, has stated that the arrival of the drug will be "a logistical, economic and clinical challenge". However, the organisation has offered to collaborate with the health authorities and the industry to guarantee early access, with pilot programmes in reference centres.
In addition to the US and Europe, the new treatment has already been approved in China, Israel, Japan and South Korea.
Publicidad
Publicidad
Publicidad
Publicidad
Esta funcionalidad es exclusiva para registrados.
Reporta un error en esta noticia

Debido a un error no hemos podido dar de alta tu suscripción.
Por favor, ponte en contacto con Atención al Cliente.

¡Bienvenido a SURINENGLISH!

Tu suscripción con Google se ha realizado correctamente, pero ya tenías otra suscripción activa en SURINENGLISH.
Déjanos tus datos y nos pondremos en contacto contigo para analizar tu caso

¡Tu suscripción con Google se ha realizado correctamente!
La compra se ha asociado al siguiente email
Comentar es una ventaja exclusiva para registrados
¿Ya eres registrado?
Inicia sesiónNecesitas ser suscriptor para poder votar.