
Sections
Highlight

SUSANA ZAMORA
Monday, 22 November 2021, 13:11
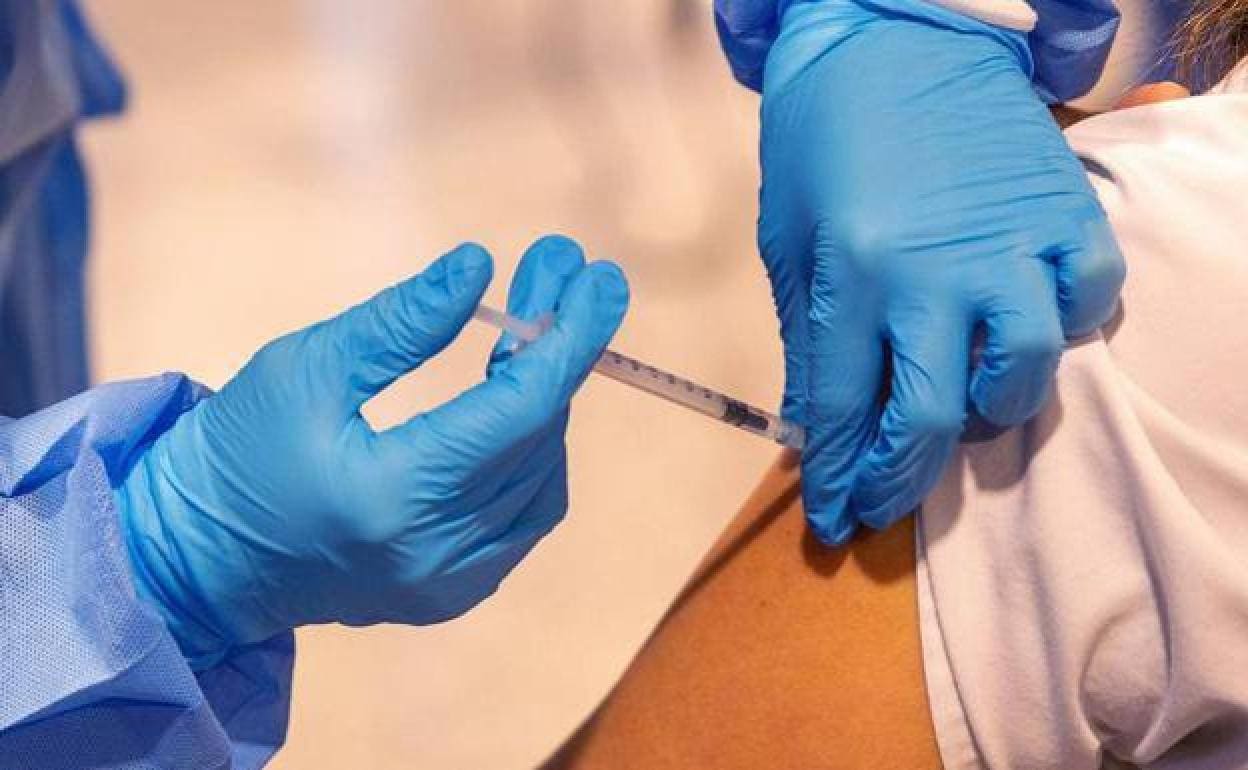
The Spanish Agency for Medicines and Health Products (Aemps) has authorised phase 2 of the clinical trials for the Covid-19 vaccine, which is being developed by the Spanish pharmaceutical company Hipra.
It is one more step in the development of the experimental vaccine, which will be carried out in ten Spanish hospitals and in which 1,075 volunteers will participate in total.
One of them is Malaga’s Regional Hospital (formerly the Carlos Haya), which has been chosen for its long research history in clinical trials.
The start of the latest trial is imminent and expected before the end of this month. Currently, the medical team are performing check-ups of the volunteers recruited, so far, to verify that they meet all the inclusion criteria for the 100 who will take part in the city. Because it is still in the screening phase (there may be people who are rejected because they do not comply with the requirements), those who wish to participate can do so by calling the mobile phone number 690 997 713 or emailing: preventiva.hrmal.sspa@juntadeandalucia.es
The medical team is looking for healthy adults (men and women), who have not had the disease, and who have received two doses of the Comirnaty vaccine (Pfizer). Six months must have elapsed since the last jab, but not more than one year. “The point is to compare two groups of people to see the safety and tolerance in the use of a third booster dose. It is a double-blind trial: neither the patient nor the research team know which vaccine is administered, whether it is Hipra or Pfizer”, explains Salvador Oña, head of the Preventive Medicine service at the Regional Hospital of Malaga and coordinator of the research team. “What we need to show is that the immune response of the Hipra vaccine is not inferior to that of Pfizer.”
In addition to the Malaga Regional Hospital, the Hospital Clínic and the Vall d'Hebron in Barcelona will also work in this phase 2 trial; the Doctor Josep Trueta University Hospital (Gerona); the Germans Trias i Pujol Hospital (Badalona); the General University Hospital Gregorio Marañón and La Paz in Madrid and the Prince of Asturias (Alcalá de Henares). Also, the Hospital Universitario de Cruces (Baracaldo) and the Hospital Clínico Universitario de Valencia.
To authorise this new phase 2 clinical trial stage, Aemps took into account that in the first phase, which it approved in August, no additional safety problems have been reported and only the expected effects in any vaccine have been found. If the results obtained from this second phase are favourable, phase 3 will begin immediately, and more hospitals from Spain and other European countries will join it, with a greater number of volunteers.
The forecast is that the vaccine, which will be kept between 2 and 8º C and, therefore, will facilitate logistics and distribution, may be available in the first half of 2022, although subject to obtaining the appropriate authorisations.
Publicidad
Publicidad
Publicidad
Publicidad
Esta funcionalidad es exclusiva para registrados.
Reporta un error en esta noticia

Debido a un error no hemos podido dar de alta tu suscripción.
Por favor, ponte en contacto con Atención al Cliente.

¡Bienvenido a SURINENGLISH!

Tu suscripción con Google se ha realizado correctamente, pero ya tenías otra suscripción activa en SURINENGLISH.
Déjanos tus datos y nos pondremos en contacto contigo para analizar tu caso

¡Tu suscripción con Google se ha realizado correctamente!
La compra se ha asociado al siguiente email
Comentar es una ventaja exclusiva para registrados
¿Ya eres registrado?
Inicia sesiónNecesitas ser suscriptor para poder votar.